
Understanding the differences between homopolymers and copolymers is essential for selecting the appropriate material for injection molding. In this article, we will delve into the characteristics, properties, and applications of both homopolymers and copolymers, providing insights into how these polymer classifications influence material selection and performance.
What is Homopolymer
A homopolymer is a type of polymer that consists of a single repeating monomer unit in its chain structure. In other words, it is made up of identical monomer molecules that are covalently bonded together to form a long polymer chain.
- A homopolymer has one type of monomer: A-A-A-A-A-A
What are the Different Types of Homopolymers?
Some key examples of homopolymers include:
- Polyvinyl chloride (PVC) – made from repeating units of vinyl chloride
- Polyethylene (PE) – made from repeating units of ethylene
- High-density polyethylene (HDPE) – a type of polyethylene with higher density and crystallinity
- Polypropylene (PP) – made from repeating units of propylene
- Polycarbonate – made from repeating units of bisphenol A and phosgene
- Polyester – made from repeating units of an ester monomer
- Nylon 6 – made from repeating units of caprolactam
- Nylon 11 – made from repeating units of 11-aminoundecanoic acid
- Polytetrafluoroethylene (PTFE) – made from repeating units of tetrafluoroethylene
- Polystyrene – made from repeating units of styrene
- Polyacrylonitrile – made from repeating units of acrylonitrile
- Nylon 6,6 – made from repeating units formed by condensing hexamethylenediamine and adipic acid
What is Copolymers?
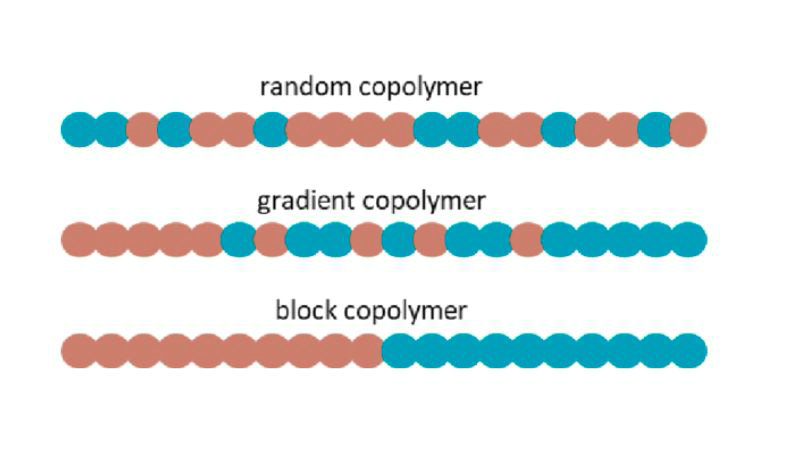
A copolymer is a type of polymer that is derived from more than one species of monomer. In other words, copolymers are made by copolymerization – the polymerization of two or more different types of monomers together in a single polymeric chain.
- A copolymer has two or more monomers bonded together: A-B-A-B-A-B
What are the Different Types of Copolymers?
Some key examples of copolymers include:
- Styrene butadiene rubber (SBR) – a random copolymer made from styrene and butadiene monomers
- Acrylonitrile butadiene styrene (ABS) – a terpolymer made from acrylonitrile, butadiene, and styrene monomers
- Ethylene-vinyl acetate (EVA) – a random copolymer of ethylene and vinyl acetate
- Polyethylene-vinyl acetate (PEVA) – a copolymer of ethylene and vinyl acetate
- Nitrile rubber – a random copolymer of acrylonitrile and butadiene, used in disposable gloves and seals
- Styrene-acrylonitrile copolymer (SAN) – an alternating copolymer of styrene and acrylonitrile
- Nylon 6,6 – an alternating copolymer of hexamethylene diamine and adipic acid
- Poly(lactic-co-glycolic acid) (PLGA) – a copolymer of lactic acid and glycolic acid
- High-impact polystyrene (HIPS) – a graft copolymer of polystyrene and polybutadiene
- Styrene-isoprene-styrene (SIS) – a block copolymer
What is the Difference Between Homopolymer and Copolymer?
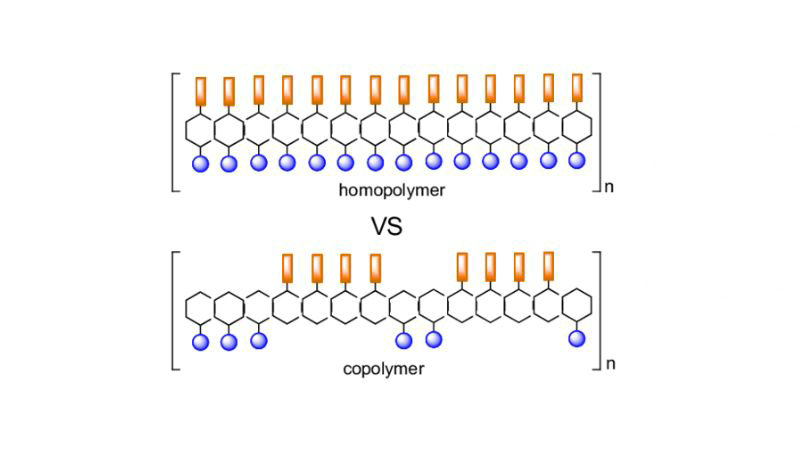
The key difference is that the homopolymer contains only one type of monomer repeating in a simple structure, while the copolymer incorporates two or more different monomers, leading to more complex structures and combined properties. The choice between them depends on the specific application requirements.
Homopolymers generally have higher crystallinity levels, resulting in superior short-term mechanical properties, including stiffness, tensile strength, impact resistance, and initial creep resistance.
On the other hand, copolymers exhibit better oxidation resistance and improved long-term creep and creep rupture resistance.
Copolymers, with their lower crystallinity, offer advantages in dimensional stability, lower friction, and reduced wear.
Although homopolymers have a lower moisture uptake, copolymers are more resistant to hydrolysis in hot water and have better resistance to alkali materials.
While homopolymers have a higher heat distortion temperature due to their higher crystallinity, copolymers boast higher continuous use temperatures because of their superior long-term stability.
Here is the form for easy understanding:
| Property | Copolymer | Homopolymer |
|---|---|---|
| Crystallinity | ↓ | ↑ |
| Stiffness | ↓ | ↑ |
| Tensile strength | ↓ | ↑ |
| Impact resistance | Higher, especially at low temperatures | ↓ |
| Creep resistance | Better long-term performance | Better short-term performance |
| Fatigue resistance | ↓ | ↑ |
| Dimensional stability | ↑ | ↓ |
| Chemical resistance | Better, especially to acids and alkalis | ↓ |
| Oxidation resistance | ↑ | ↓ |
| Water resistance | Better in hot water | Lower moisture uptake, but less resistant to hydrolysis |
| Temperature resistance | Higher continuous use temperature due to better long-term stability | Higher heat distortion temperature, but lower continuous use temperature |
| Processing | Lower processing temperature and wider processing window due to lower crystallinity | Narrower processing window and higher processing temperature due to higher crystallinity |
| Glass fiber reinforcement | Stronger mechanical properties when glass-filled due to better coupling | Weaker mechanical properties when glass-filled compared to copolymer |
What are the Applications of Homopolymers and Copolymers?
By understanding the applications of homopolymers and copolymers, you can easily decide which one you should choose in a given situation.
| Application | Homopolymers | Copolymers |
|---|---|---|
| Packaging | Plastic containers, bags, films for food and commodities (e.g., polyethylene, polypropylene) | Ethylene vinyl alcohol (EVOH) as barrier layers in food packaging; ethylene-vinyl acetate (EVA) in adhesives and sealants |
| Medical and Healthcare | Medical devices, syringes, surgical instruments, disposable medical supplies (e.g., polypropylene, PVC) | Biocompatible copolymers like PLGA in medical implants, drug delivery systems, tissue engineering; block copolymers in wound dressings and medical devices |
| Automotive | Car interiors, fuel tanks, battery cases, bumpers, interior trim, instrumental panels (e.g., polypropylene) | Ethylene copolymers in gaskets, hoses, and interior trim for durability and flexibility; block copolymers like SBS in tires |
| Textiles | Fibers and fabrics for carpets, upholstery, clothing, ropes, twine (e.g., polyester, polyamide) | Spandex and nylon-6,6 for moisture-wicking, flame resistance; acrylic copolymers in cosmetics and personal care products |
| Electrical Components | Cable insulation, connectors, capacitors (e.g., polyethylene, PTFE) | – |
| Construction | Pipes, fittings, insulation materials, siding (e.g., PVC) | Ethylene copolymer-based hot-melts in building and construction adhesives |
| Consumer Goods | Toys, sports equipment, furniture, appliances, luggage, housewares (various homopolymers) | Block copolymers in footwear, toys, and other consumer goods |
| Agriculture | Irrigation pipes, silage baling, ground moisture retaining goods, greenhouse films (e.g., polyethylene) | – |
| Industrial | Acid and chemical tank sheets, pipes, returnable transport packaging (various homopolymers) | Membranes for gas and liquid separation; emulsifiers and dispersants |
| Advanced Materials | – | Block copolymers in composites, hybrid materials, and responsive materials; self-assembled nanostructures for various applications |






