What is Electroplating

Electroplating is the process that coats materials with a thin layer of metal. At its core, electroplating uses electric current to deposit metal ions onto a surface.
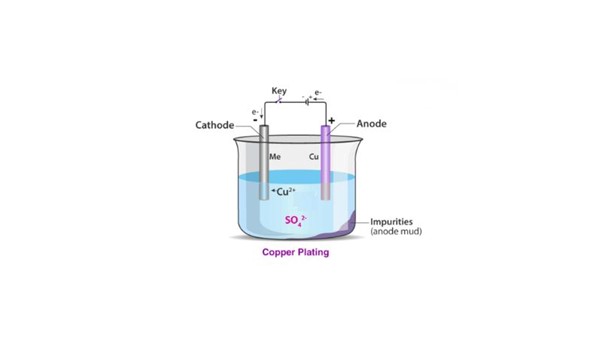
The process of electroplating involves placing the object to be coated and a piece of metal in a solution called an electrolyte. When an electrical current passes through the solution, metal ions move from the metal piece to the object, creating a thin, even layer.
Key Components:
- Anode: The positive electrode is usually made of metal intended for plating.
- Cathode: The negative electrode, the object to be plated.
- Electrolyte Solution: Contains metal ions and allows current to flow.
Benefits of Electroplating
- Corrosion Resistance: Electroplating enhances metals’ corrosion resistance, protecting them from environmental damage.
- Aesthetic Appeal: It provides a decorative finish, improving product appearance, especially in jewelry and automotive parts.
- Improved Wear Resistance: Electroplated coatings increase hardness and wear resistance, extending component lifespan and reducing maintenance.
- Conductivity and Performance: Electroplating boosts electrical conductivity, making it vital for electronics and electrical applications.
Manufacturing Process of Electroplating
The electroplating process includes three main steps: clean the surface, set up the electroplating system, and apply the electrical current.
Preparing the Surface
The first step in electroplating is cleaning the object. Dirt, oil, and rust must be removed to ensure the coating sticks properly. Cleaning is done using solvents, acids, or abrasives. Mechanical cleaning, such as sandblasting or wire brushing, is often used to smooth the surface.
After cleaning, the object might undergo an activation step. This involves dipping it into an acid solution to remove thin oxide layers. This ensures the metal sticks to the surface during electroplating.
Setting Up the Electroplating System
This involves creating a setup where the object becomes the cathode while the plating metal is the anode. Both are dipped into a solution called an electrolyte. The electrolyte contains dissolved metal ions from the anode.
The setup also includes a power supply to allow the flow of electrical current. The voltage and current need careful adjustment to control the coating thickness and quality.
Applying the Electrical Current
The actual plating happens when the electrical current is applied. When the current flows through the solution, the metal ions move towards the cathode. They deposit onto the object, forming a smooth, even layer.
The current density, or the amount of current passing through a specific area, affects the plating quality. Too high or too low current density can lead to issues such as rough surfaces or uneven thickness.
The duration of the process also determines the layer’s thickness. Longer plating time means a thicker coat.
Types of Electroplating and How to Choose?
Different types of electroplating methods include barrel, rack, and continuous plating.
Barrel Plating
Barrel plating is suitable for small items. Objects are loaded into a barrel made of non-conductive material. The barrel rotates and immerses in a plating solution.
This movement ensures even coating on all items, which is cost-effective and can handle a large volume at once. Barrel plating is ideal for parts like nuts and bolts, where precision is less critical. The challenge of barrel plating is that it might not equally coat very intricate or detailed items due to their complex shapes.
Rack Plating
Rack plating is designed for larger or more delicate parts. Each item is attached to a rack that holds it in place during plating, ensuring a uniform finish.
This method offers more control over the plating process. It is used when detail or appearance is important, like in automotive or aerospace parts. While it takes more time and costs more than barrel plating, rack plating provides superior precision and is versatile for different materials.
Continuous Plating
Continuous plating is used for high-volume production of coils, wires, or strips. The material continuously moves through a plating solution.
This method is efficient for producing long lengths in a short time. It’s suitable for industries like electronics, where large quantities of uniform coated material are needed. Continuous plating ensures consistent quality but requires a significant initial investment in machinery and setup. It’s ideal for items that demand consistent thickness and quality over long surfaces.
Materials and Chemicals Used
Electroplating involves using specific metals, solutions, and electrodes. The choice of materials affects the plating’s quality, durability, and appearance.
Metals Commonly Used
The choice of metal depends on the desired results, whether for decorative purposes or functional applications.
Metals like nickel, copper, chromium, gold and silver are frequently chosen for electroplating.
Gold is valued for its conductivity and resistance to tarnish. Silver is used for its excellent conductivity and aesthetic appeal. Nickel and copper add layers for corrosion resistance and conductivity, while chromium provides a shiny.
Solutions and Electrolytes
As we talked before, the electroplating process requires a solution called an electrolyte. This solution contains dissolved metal ions.
Nickel plating, for example, uses a nickel sulfate solution. A copper sulfate solution is standard for copper plating.
Here is a chart to help you get a glance:
| Metal Plated | Electrolyte Solution | Common Use |
| Nickel | Nickel Sulfate (NiSO₄) | Decorative and functional coatings |
| Copper | Copper Sulfate (CuSO₄) | Electrical components, plumbing |
| Chromium | Chromic Acid (H₂CrO₄) | Decorative finishes, corrosion resistance |
| Zinc | Zinc Sulfate (ZnSO₄) or Zinc Chloride (ZnCl₂) | Corrosion protection for steel |
| Gold | Gold Chloride (AuCl₃) or Gold Cyanide (K[Au(CN)₂]) | Jewelry, electronics |
| Silver | Silver Nitrate (AgNO₃) or Silver Cyanide (AgCN) | Jewelry, mirrors, electronics |
| Tin | Tin Sulfate (SnSO₄) | Coatings for corrosion resistance |
| Lead | Lead Acetate (Pb(C₂H₃O₂)₂) | Battery terminals, decorative items |
| Palladium | Palladium Chloride (PdCl₂) | Electronics, decorative applications |
| Rhodium | Rhodium Chloride (RhCl₃) | Automotive parts, decorative finishes |
Advancements in Electroplating Technology

Electroplating technology continues to improve. New chemicals are making the process safer and more efficient. Automation is also playing a significant role in improving accuracy and speed. New uses for electroplating are constantly being discovered.
Innovation in Electroplating Chemicals
Recent advances in chemical formulations are making electroplating more effective and environmentally friendly. Bio-based plating additives are now being used to reduce toxic waste.
Nanotechnology is also helping to develop smaller, more precise materials. These new chemicals increase durability and make the plating process safer for workers and the environment.
Non-cyanide alternatives are replacing cyanide baths, which are harmful. They offer a safer approach without compromising quality. Additives that enhance brightness and smoothness are becoming more common. These improvements result in a better-finished product and reduced costs.
Process Automation
Automation in electroplating is transforming the industry. Automated systems ensure consistent quality and reduce the chance of human error. Robots can manage complex plating cycles with precise timing.
These systems save time and labor. By monitoring every aspect of the plating process, such systems provide detailed data analysis and control.
Plating lines can be adjusted in real-time to optimize performance. This leads to more efficient use of resources and fewer defects. Automated plating reduces waste and increases productivity, making it a popular choice for manufacturers looking to stay competitive.
Applications of Electroplating
| Application Area | Description |
| Jewelry | Applies a thin layer of precious metals (e.g., gold, silver) to enhance appearance and value. |
| Automotive Industry | Used for components like bumpers and trim to improve corrosion resistance and aesthetics. |
| Electronics | Essential for manufacturing electronic components, enhancing conductivity and solderability. |
| Aerospace | Applied to critical components for durability, wear resistance, and corrosion protection. |
| Household Items | Used on items like cutlery and faucets to enhance appearance and resistance to tarnishing. |
| Medical Devices | Enhances biocompatibility, corrosion resistance, and ease of sterilization for surgical instruments and implants. |
| Telecommunications | Used in connectors and antennas to improve conductivity and reduce signal loss. |
| Battery Manufacturing | Improves conductivity and performance of battery electrodes in rechargeable batteries. |
Work With Moldie
Moldie provides different metal surface treatments, offering a range of solutions to enhance the durability, appearance, and functionality of metal products. Our expertise includes various surface treatment processes, such as electroplating, anodizing, powder coating, and more, tailored to meet the specific needs of different industries.
Don’t compromise on quality or performance. Choose Moldie as your partner for metal surface treatment and experience the difference that expertise, quality, and customer care can make.
Frequently Asked Questions
What are the safety considerations when performing electroplating?
Safety in electroplating involves wearing protective gear like gloves and goggles. Care should be taken to ventilate the area well because of the fumes. It is important to handle chemicals properly to avoid spills and exposure.
How does the electroplating process prevent corrosion on metals?
Electroplating prevents corrosion by placing a metal layer over another surface. This acts as a barrier, protecting the base metal from environmental factors that can cause rust or degradation.
What factors influence the rate of deposition during electroplating?
The rate of deposition is influenced by factors like current density, temperature of the solution, and the concentration of metal ions in the plating solution. The distance between electrodes can also impact the rate.






