Understanding the fundamentals of copolymer chemistry is crucial for anyone interested in polymer science, materials engineering, or related fields. This article will delve into the definition, classification, synthesis, properties, and applications of copolymers, providing a comprehensive overview of these fascinating macromolecules that have revolutionized modern materials science.
What are Copolymers?
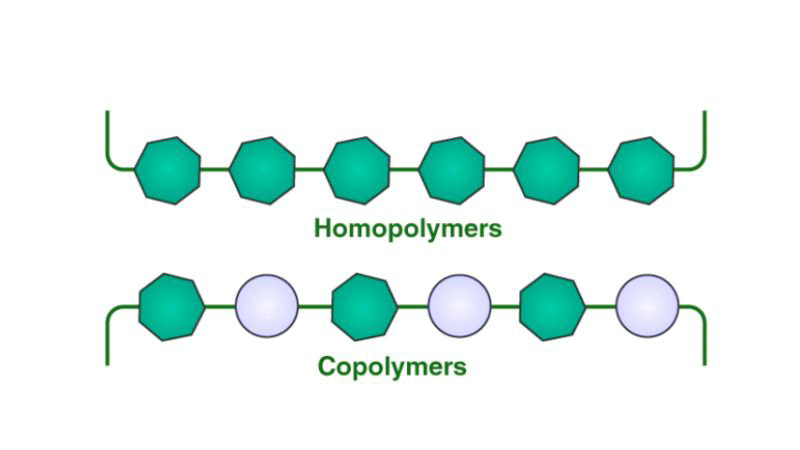
A copolymer is a type of polymer composed of two or more different types of monomers, which are the basic building blocks of polymers. These monomers are chemically bonded to form long chains during the process of polymerization.
Copolymers feature a combination of two or more monomers that can be arranged in various patterns. These patterns include alternating, random, block, and graft structures, each of which imparts distinct chemical properties and characteristics to the material.
How Copolymers are Made?
Copolymers are synthesized by polymerizing two or more different monomers together using techniques like addition polymerization (free radical, anionic) or condensation polymerization.
The choice of monomers, their ratio, polymerization method and processing determines the final copolymer structure and properties.
- Addition polymerization – monomers with reactive groups (often double carbon bonds) are linked together in a chain. This includes free radical and anionic polymerization. Initiators are used to start and control the reaction.
- Condensation polymerization – monomers with functional groups (often ester or amide groups) react to form a polymer, generally with the elimination of a water or methanol molecule. Catalysts are employed to control the reaction.
What is anionic polymerization and radical polymerization?
- Anionic Polymerization:
Anionic polymerization is an ionic chain-growth polymerization initiated by nucleophilic reagents such as organolithiums, Grignard reagents, and metal alkoxides.
It involves the polymerization of vinyl monomers possessing electron-withdrawing groups like methyl methacrylate, acrylonitrile, 2-vinylpyridine, as well as conjugated monomers like styrene and 1,3-butadiene.
The polymerization proceeds with the growing chain end carrying a negative charge and a countercation.
Anionic polymerization can be “living” if there are no termination or chain transfer steps, allowing control over molecular weight and enabling block copolymer synthesis.
- Radical Polymerization:
Radical polymerization, or more specifically free-radical polymerization, forms polymers from vinyl monomers through radical reactions involving free radical intermediates.
It is initiated by free radicals generated from radical initiators and proceeds through propagation steps where radicals react with monomers to grow the polymer chain.
Monomers that readily undergo radical polymerization include styrenes, (meth)acrylates, (meth)acrylamides, and acrylonitrile which can stabilize the propagating radicals.
Conventional radical polymerization has little control over molecular weight and dispersity. Controlled/living radical polymerization methods like ATRP provide better control.
What are the Different Types of Copolymer
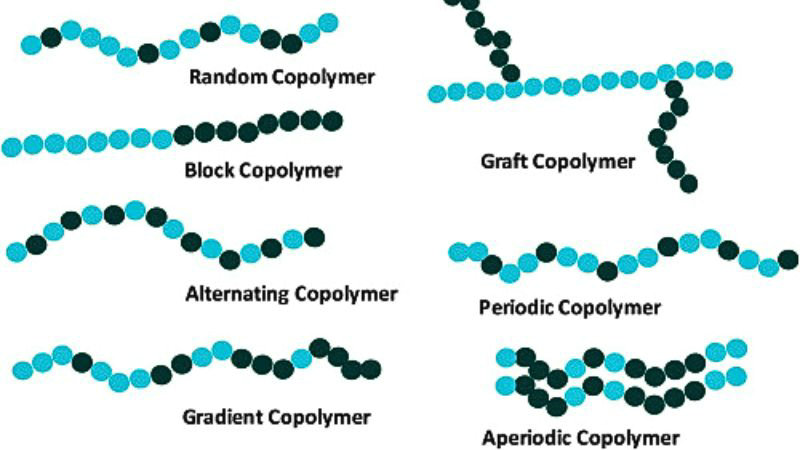
Two main types of copolymers are linear copolymers and branched copolymers.
Linear copolymers consist of a single main chain with the different monomer units arranged along that chain. They are further classified into:
- Alternating copolymers – alternating copolymers mean the two monomer units alternate in a regular pattern, e.g. (-A-B-A-B-)n
- Statistical copolymers – statistical copolymers (also known as random copolymers) are one type of linear copolymer. The monomer units are distributed randomly along the chain, following statistical rules
- Block copolymers – composed of blocks of each monomer type covalently bonded together, e.g. -A-A-A-B-B-B-
- Gradient copolymers – composition gradually changes along the chain
- Periodic copolymers – the monomer units are arranged in a repeating sequence, for example (A-B-A-B-B-A-A-A-A-B-B-B)n
Branched copolymers have a main chain with one or more polymeric side chains attached to it. The two main types are:
- Graft copolymers – side chains are structurally distinct from the main chain
- Star-shaped copolymers – multiple polymer chains radiate from a central core
- Brush copolymers – with a high density of polymeric side chains attached to a linear backbone, leading to a worm-like or cylindrical brush structure
- Comb copolymers – consisting of a linear backbone with a lower density of polymeric side chains, resulting in a more flexible comb
What are the Advantages of Copolymers?
Copolymers offer a wide range of advantages over homopolymers, including tunable properties, improved mechanical and chemical properties, cost-effectiveness, and enhanced compatibility.
| Advantage | Description |
|---|---|
| Tunable properties | Properties can be customized by adjusting monomer proportions and arrangement |
| Improved mechanical strength and chemical resistance | Exhibit properties not achievable with homopolymers |
| Cost-effectiveness | Can substitute for metals or more complex materials |
| Enhanced compatibility | Improve compatibility between otherwise incompatible materials |
| Novel materials | Copolymerization can result in unique materials |
| Good physical properties | Flexibility, elasticity, and rigidity can be tuned |
| Easier processing | Lower processing temperature and wider processing window |
| Better long-term performance | Superior thermal stability, oxidation resistance, and creep resistance |
What are the Disadvantages of Copolymers?
Copolymers also have some drawbacks related to manufacturing complexity, less predictable properties, higher costs, potential degradation, and weaker mechanical properties.
| Disadvantage | Description |
|---|---|
| Complex manufacturing | Copolymerization is more complex due to managing multiple materials with different reactivity rates. |
| Less predictable properties | Achieving specific properties in copolymers may be less predictable |
| Higher costs | Using multiple monomers and additional process complexity increases production costs. |
| Compatibility issues | Some monomers are incompatible, preventing or complicating the establishment of certain copolymers. |
| Weaker mechanical properties | – |
| Lower temperature resistance | – |
What are the Copolymer Examples in Industry

Styrene-Based Copolymers:
- Acrylonitrile Butadiene Styrene (ABS): Used primarily in the automotive and electronics industries, ABS is appreciated for its toughness and impact-resistant properties.
- Styrene-Isoprene-Styrene (SIS): This copolymer is often found in adhesives and sealants, offering good elasticity and strength.
Ethylene-Based Copolymers:
- Ethylene-Vinyl Acetate (EVA): Known for its rubber-like softness and flexibility, EVA is widely used in food packaging and as a film for laminating floors.
- Polyethylene Tetrafluoroethylene (ETFE): ETFE is chosen for its high melting point and excellent electrical properties, making it suitable for wire coatings and as a lightweight substitute for glass.
Frequently Asked Questions
Are copolymers the same as polymers?
No, all copolymers are polymers, but not all polymers are copolymers. Copolymers are a subclass of polymers characterized by having two or more different repeating monomer units, which provides them with different structures and properties compared to homopolymers consisting of only one type of monomer.
What is the difference between homopolymer and copolymer?
Homopolymers contain a single type of monomer repeating in a simple chain, while copolymers have two or more different monomers arranged in a more complex structure. This leads to differences in their synthesis, properties and end-use applications
Are copolymers safe for the skin?
Sodium acrylates copolymer and related acrylates copolymers have been studied and considered safe for cosmetic use “when formulated to avoid irritation.”
Acrylic acid can be severely irritating and corrosive to the skin, eyes, and respiratory tract at high exposures.
Methacrylic acid is restricted in Canadian cosmetics and classified as potentially toxic or harmful.
For what applications are copolymers commonly used?
Copolymers are utilized in a wide array of products, including automotive parts, plastic containers, and medical devices due to their customizable mechanical and chemical properties.
In what way do random copolymers differ from other copolymer structures?
Random copolymers contain a mix of monomer units arranged in no particular order along the chain, resulting in polymers with a balance of properties from the constituent monomers, such as improved impact resistance or flexibility.
How does the composition of a copolymer influence its properties?
The ratio and arrangement of monomers within a copolymer directly affect its thermal, mechanical, and chemical properties, meaning the material can be engineered for specific functions, like increased elasticity or resistance to solvents.






